


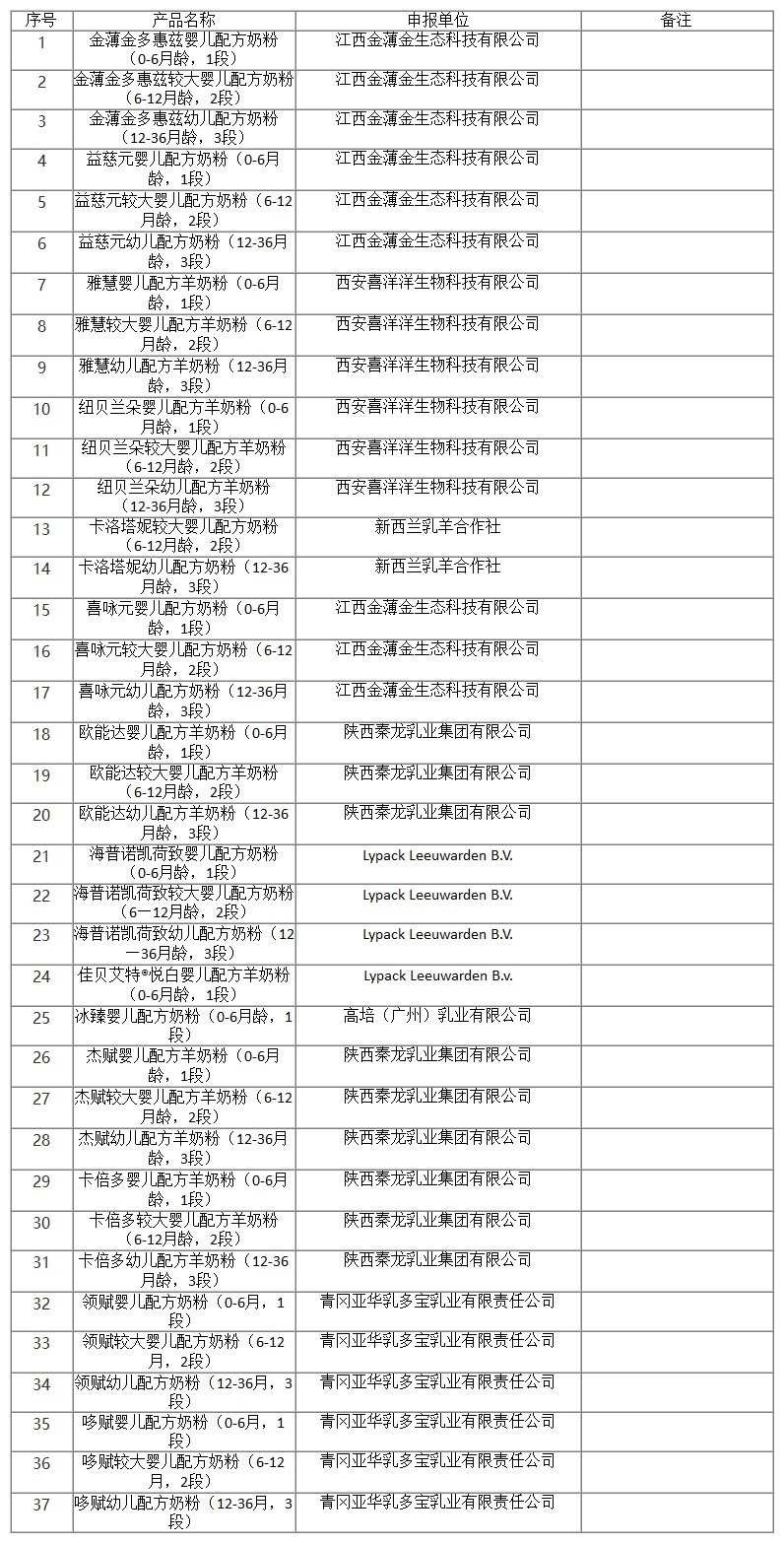
On October 20, 2022, the Center of Food evaluation of the State Administration for Market Regulation updated the list of approval document (decision) for the registration of infant formula product, which includes 37 kinds of infant/older infant/young children formula products, decision details as below: Jinbojin Duohuizi infant formula (0-6 months, stage 1), Jinbojin Duohuizi older infant formula (6-12 months, stage 2), Jinbojin Duohuizi young children formula (12-36 months, stage 3),etc.
国家市场监督管理总局食品审评中心发布2022年10月20日婴幼儿配方乳粉产品配方注册批件(决定书)待领取信息,涉及金薄金多惠兹婴儿配方奶粉(0-6月龄,1段),金薄金多惠兹较大婴儿配方奶粉(6-12月龄,2段),金薄金多惠兹幼儿配方奶粉(12-36月龄,3段)等37种婴幼儿配方乳粉类产品。
Need help or have a question?
Send mail